Multilingual patient recruitment in clinical trials has become a decisive factor in ensuring the success of any study. With the arrival of new FDA and EMA regulations in 2025, participant-facing materials —from informed consent forms to plain-language summaries and eConsent platforms— must be available in multiple languages, with digital accessibility and readability tailored to each profile. Investing in professional medical translation and accessibility from the protocol design stage not only ensures regulatory compliance but also accelerates patient enrollment and strengthens trust in the clinical research process.
Why can’t trials afford monolingual outreach anymore?
The 2022 Food & Drug Omnibus Reform Act (FDORA) made Diversity Action Plans (DAPs) legally binding, and the FDA’s draft guidance (to be finalised mid-2025) spells out that each plan must explain how patient-facing materials will be delivered “in languages material to enrolment goals.” In the EU, Regulation 536/2014 already obliges sponsors to publish plain-language lay summaries in every participant language within 12 months of database lock.
Decentralised & hybrid trial models amplify the stakes: every SMS reminder, chatbot, or eConsent screen must let patients switch language, resize text, or invoke text-to-speech—or sponsors risk protocol deviations and IRB queries. A March 2025 DIA Global Forum survey lists “language & accessibility gaps” among the top-10 operational constraints in DCTs.
Bottom line: recruitment speed, compliance, and patient trust now hinge on getting language, readability, and accessibility right at protocol design—not after IRB submission.

What’s new in the 2025 rule-set?
- US FDA
- Key 2025 requirement: DAP must include language-access provisions for under-represented groups. Draft guidance due for finalisation June 2025.
- Why it matters: IRBs can reject plans that omit localisation, delaying first-patient-in (FPI).
- EU/EMA
- Key 2025 requirement: Lay summaries in all participant languages + readability targets (Grade 6-8).
- Why it matters: Multilingual plain-language writing becomes audit-traceable.
- Accessibility
- Key 2025 requirement: WCAG 2.2 AA & Section 508 for all digital recruitment assets.
- Why it matters: eConsent or DCT apps that lack captions or alt-text can trigger protocol amendments.
Where do sponsors still struggle with multilingual patient recruitment?
1. Late Localization Request
- Impact: Adds 4-6 weeks for back-translation and IRB re-review.
- Real-world Example: 12 ICF (Informed Consent Form) versions were resubmitted after the Institutional Review Board (IRB) flagged non-English sites.
2. Over-reliance on Raw Machine Translation (MT)
- Impact: Leads to glossary gaps, quality holds, and necessary amendments.
- Real-world Example: A drug-interaction term was mistranslated in a Hausa pilot site.
3. Ignoring Accessibility
- Impact: Non-compliant eConsent pauses deployment.
- Real-world Example: A dashboard lacked captions and screen-reader tags.
4. Fragmented Version Control
- Impact: Results in inconsistent risk language across different communication channels.
- Real-world Example: Flyers, SMS messages, and chatbot communications carried divergent warnings.
DIA’s 2025 list confirms that language and accessibility rank among the top-10 Decentralized Clinical Trial (DCT) bottlenecks.
Build language & accessibility into the protocol timeline
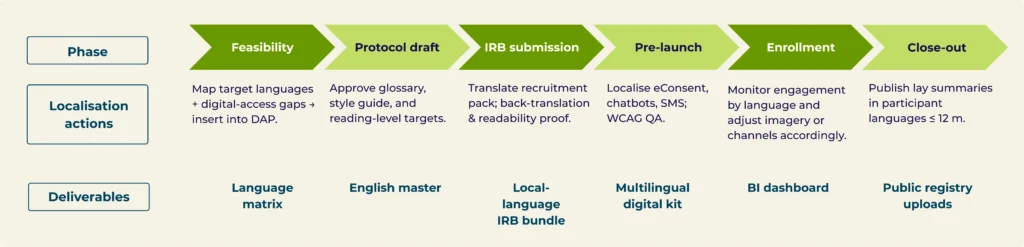
Mini case — Phase III Oncology: 12 countries, 18 languages, FPI five weeks early
The challenge.
A global sponsor needed to enroll 900 under-represented patients across the U.S., Brazil, and the EU. Regulators required a Diversity Action Plan that explicitly mapped recruitment and consent materials in 18 languages, with plain-language copy and WCAG-compliant digital assets. Timelines were tight and first-patient-in (FPI) was at risk.
What we did.
In week 0, our medical writers rewrote the master pack in plain language (≈ Grade 6–8 reading level) and established a shared glossary and style guide. Within 48 hours we produced a neural-MT first pass with locked terminology, then ran in-country clinical validation and back-translation with reconciliation for high-risk content (ICF and safety alerts). In parallel, we added the accessibility layer (tagged PDFs, captions/subtitles, alt-text ≤ 125 characters, text-to-speech) and packaged everything for the eConsent platform as responsive HTML + WebVTT. A central version-control hub pushed any protocol “delta” to all languages within 24 hours.
The outcome.
- Full 18-language recruitment kit delivered in 14 days (vs. 20-day historical baseline).
- ≈30% cycle-time reduction thanks to the AI + human model.
- IRB approval on first submission with zero linguistic observations.
- FPI achieved five weeks earlier than the contractual date.
Key takeaways.
Bake the language matrix into the DAP from the first draft, pair clinician review with plain-language rewriting, treat accessibility as non-negotiable, and integrate with the DCT/eConsent stack before IRB—those four moves protect timelines and diversity goals.
FAQ about Multilingual Patient Recruitment
Q1. How many languages do we really need?
Cover the top patient languages at each site plus any minority groups critical to diversity targets—that’s the FDA’s litmus test for “material” languages in a DAP. (U.S. Food and Drug Administration)
Q2. Will IRBs accept AI-generated translations?
Yes—if you document qualified post-editing, clinician review, back-translation, and version control. Raw MT rarely passes ethics review.
Do you want to improve your Multilingual Patient Recruitment materials and comply with FDA/EMA requirements without delays?
Let’s make every patient—in every language—feel truly welcomed into your trial!
Book a free 15-minute consultation on your current materials.


